Three-D printing allows you to print fully customised complex models, reducing costs and speeding up production.
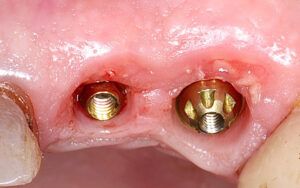
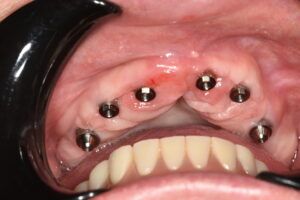
Surgical guides are among the products made by additive manufacturing. The increasing demand for these templates has led to more stringent controls, since they are medical devices that must meet the same requirements as other similar products.
To manufacture surgical guides, technicians not only need the technology and knowledge required to design and print them, but must also obtain regulatory certificates to show that the product is reliable and has been responsibly and safely manufactured. At AVINENT, our main priority is to provide the ideal product to improve the quality of the treatment offered. For this reason, it is essential that the products we manufacture and distribute are in full regulatory compliance. The regulations applicable to medical devices require that all products manufactured and distributed are monitored to detect any potential risks that may arise from their use, thus preventing possible adverse events.
Medical devices are divided in four product classes, based on the vulnerability of the human body, and AVINENT surgical guides are grouped under class IIa. Different evaluation procedures are applied by the notified body for each of these product classes. Surgical guides constitute a special group because they are intended for single, temporary (<60 min) use by a particular patient with certain characteristics.
The keys to choosing the right surgical guide
 |
MANUFACTURING LICENSE OF THE PATIENT-SPECIFIC MEDICAL DEVICE This, in the case of AVINENT, includes implants, plates and prostheses for bone reconstruction and fixation, and surgical guides (sterilised or non-sterilised). This type of licence allows AVINENT to manufacture and sell medical devices that are certified to meet quality standards and the requirements for use. |
 |
MATERIAL In AVINENT, we make our surgical guides out of polyamide, which is classed as a biocompatible material under the USP biological test and has magnificent mechanical properties, very high tenacity, sterilizable, and with excellent sliding and abrasion resistance properties. AVINENT obtains an analysis certificate for each batch of material before it is used, in order to verify that it complies with the technical specifications. |
 |
MANUFACTURING CONTROL The entire process, from the computer-based design to the manufacturing stage, must be certified. Thorough quality controls are performed during the manufacturing process, with periodic calibrations and reviews of the status and operation of all the technical equipment, together with ongoing biological controls and validated cleaning and thermal disinfection processes. This ensures that the manufacturing method, the technology, and the environment where the guides are made meet strict standards of quality and cleanliness. |
AVINENT, therefore, meets all technical-sanitary requirements for the manufacture of patient-specific 3D printed polyamide surgical guides (for cutting and guiding or positioning).
Users of medical device need to know how important it is that these products are regulated, meet essential safety and quality requirements, and provide the required performance standard and consumer information.